- Services
Services
Inoviem provides a full range of services from drug discovery to clinical development by leveraging its platforms and direct access to human pathological specimens.
All the platforms and services are label-free with no modification of the original molecules.Unravel disease relevant Mode of Action (MoA) of your molecule directly on human tissues.
Identify and validate targets in your study model or in Human.
Thanks to our ex-vivo pharmacology expertise we identify the best disease and patients subgroups.
Using patients’ samples and PIMS® technology, we identify those individuals who can benefit best from your compound as soon as the early stage of preclinical studies.
Compounds of interest can be profiled for their drugability based on their mode of action and efficacy.
Translational pharmacology empowers your ability to identify biomarkers and advance clinical developments.
Advanced SPR and proteomics drive precise analysis of biomolecular interactions and protein landscapes.
Meticulous collection and biobanking of human samples fuel robust, translational research.
- Platforms
Platforms
Inoviem provides breakthrough protein technologies for each phase of the drug development process.
One of the major advantages of our label-free technologies used under physiological conditions and in human tissue – NPOT® and PIMS® – is that the test compound retains its original structure, as well as the exact same molecular structure that would be used in therapyPIMS®
Designed to study molecular interactions and predict therapeutic responses in biomedical research
Biomarkers discovery
Methodology for the proteomic profiling of human individuals in the context of translational evaluation of biomarkers and clinical development of new drug candidates.
- Knowledge centre
- About us
About us
Inoviem Scientific is a Bioanalytical R&D company established in 2011 in Strasburg – France.

Our mission
Our mission is clear: to get the right treatment to the right patients within the right therapeutic window.
- Contact us
Schizophrenia is a devastating disease with positive and negative symptoms. Up to date, except symptomatic treatments, there are no cures available. There are strong yet not convincing indications for involvement of dopaminergic (D2 receptor), glutamatergic (Glu1 and Glu2 receptors) and serotoninergic (5-HT2A receptors) neurotransmitter in this pathophysiology.
Clozapine (Leponex) is the only effective treatment for resistant schizophrenic patients with negative symptoms (30% responders). It is widely recognized as one of the most clinically effective agents although it is associated with life threatening side effects like agranulocytosis or milder pathologies like weight gain, type 2 diabetes and cardiovascular complications. Beside there are no indications regarding Clozapine’s peripheral targets responsible for its side effects.
Clozapine has been shown to bind at least 12 different GPCRs from alpha adrenergic, dopaminergic, histaminergic, serotoninergic receptors. Early antipsychotic such as Chlorpromazine and Haloperidol have pointed towards altered dopamine D2 receptor activity since they are able to block dopamine D2 receptors.
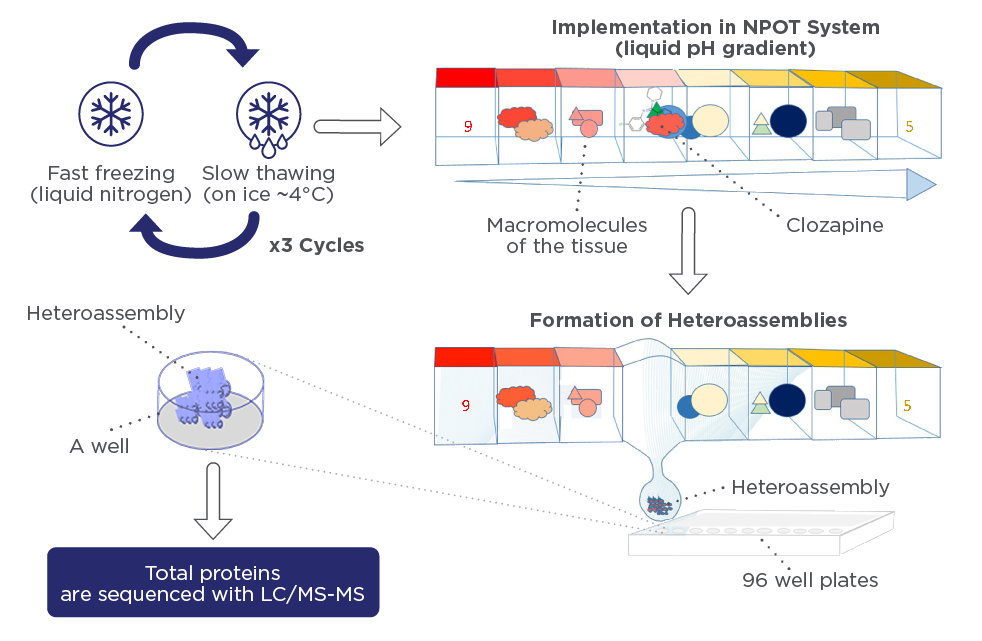
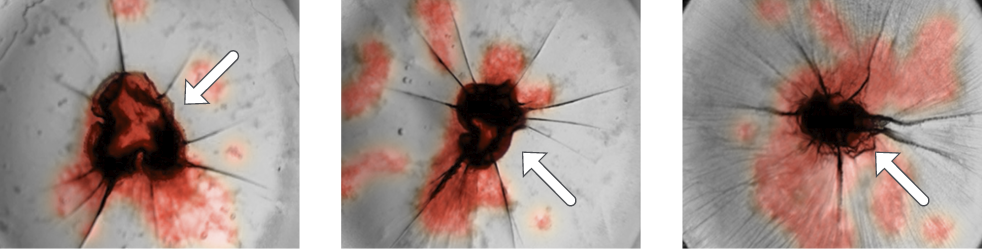
Figure 2: Formation of heteroassemblies (blue arrow) in presence of Clozapine in 3 different triplicates.
Heteroassemblies were isolated using a macroscope and sequenced by LC/MS-MS. The results are summarised in table 1
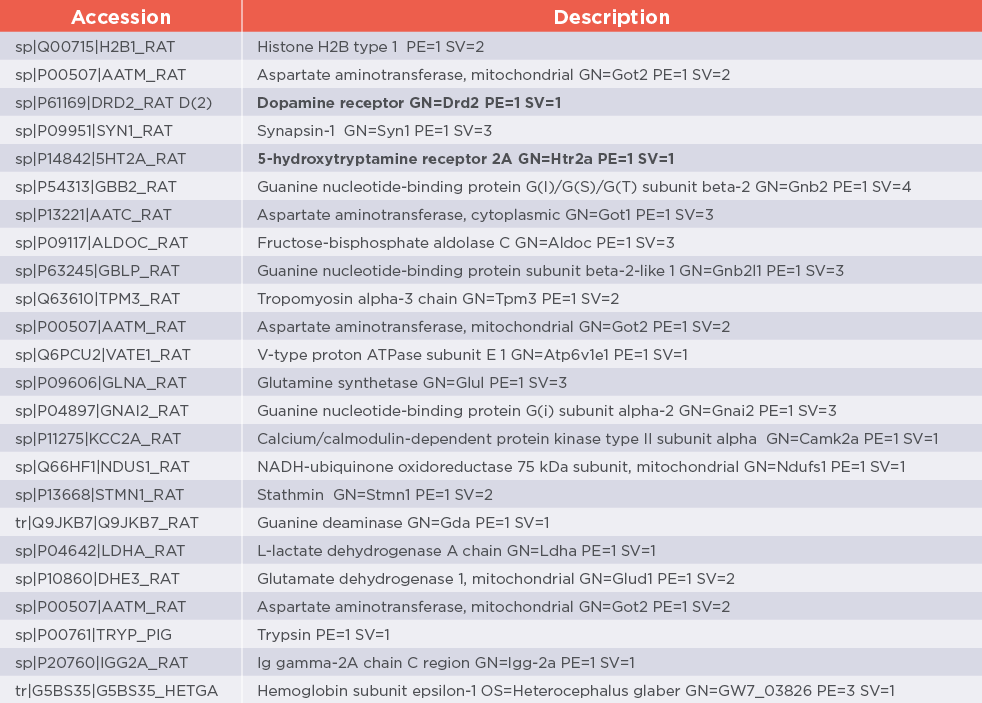
Inoviem Scientific has identified central ON Targets
Using NPOT on PBMCs from 40 patients with schizophrenia 20 of which treated with Clozapine and 20 with other antipsychotics did reveal three target(s) probably responsible for agranulocytosis, type II cardiomyopathy. The central target is a hob-protein forming hetero-oligomerisation with serotoninergic 5-HT2A and dopamine D2 receptors in rat brain homogenates (figure 2). The hob-protein is also responsible for the recruitment of several GPCRs. Clozapine interacts with the identified hob-protein (NCBI GI, 24XXX03 and PDB ID 3XFX), therefore it is plausible that latter results in conformational changes of dopamine D2 and serotoninergic 5-HT2A receptors affecting their activity (figure 3 and 4).
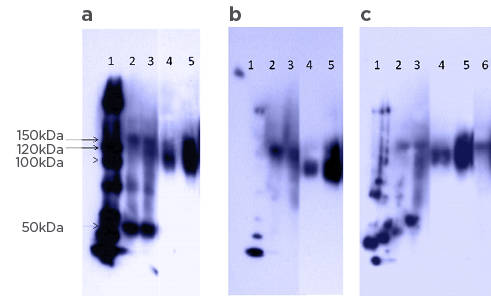
Figure 3: Native Western Blot on rat brain homogenates A) anti HOB-protein antibodies isolated from rat total brain proteins in hetero-oligomerisation with B) anti serotoninergic 5-HT2A antibodies and C) anti dopamine D2 receptors antibodies.
Lane 1: molecular ladder, lane 2: whole brain extract, lane 3: flow through, lane 4: first fraction eluted with Clozapine, lane 5: second fraction eluted with clozapine
The nature and the physiological importance of the identified target (NCBI GI, 30XXX4 and PDB ID 3XGX) explain clearly clozapine’s side effects (figure 5). It explains not only the mechanism of action for agranulocytosis but also for type II diabetes, obesity and cardiovascular complications. Structural studies at its binding sites have revealed not only the pharmacophore but also its mode of action on the peripheral target.
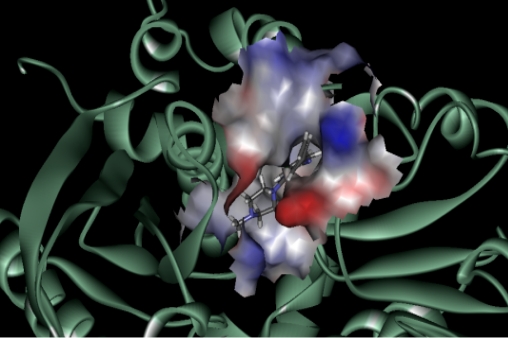
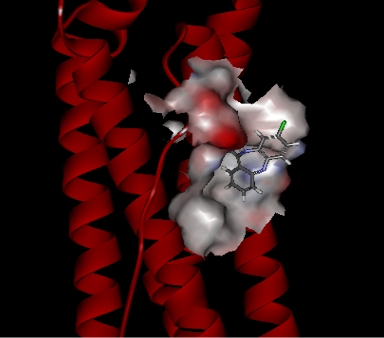
CONCLUSION
NPOT was able, to isolate from rat brain homogenates two of major Clozapine targets. We have also identified three proteins probably involved in OFF target effects and other proteins functioning as a molecular hub, modulating several GPCRs hetero-oligomerisation and activities (data not shown).
REFERENCES
Miyamoto S. et al., Mol.Psychaitry., 2005;10:79-04.
Lee T. and Tang SW., Psychiatry Res., 1984;12:277-85.
Ferré S. and Artigas F., Neuroscience Lett., 1995;187(1):61-4.
Vollenweider F.X. et al., Neuroreport 9., 1998;3897-902.
Geyer M.A. and Vollenweider F.X., Trends Pharmacol., 2008;Sci.29, 445-53.
